COVID-19 Vaccine Q&A
It’s May 2021, and while we’re still talking about COVID-19, it’s a much different conversation than it was in May 2020. Vaccines are available to anyone age 16 and older in the U.S., and as I write this, 50% of adults have had their first vaccine dose. That’s amazing progress, but the pandemic isn’t over yet. Here are a few frequently-asked vaccine-related questions and answers in the hopes that they will help anyone with lingering concerns.
- Can I still get COVID after being vaccinated?
While the COVID-19 vaccines provide very good protection against infection, and particularly severe COVID-19 illness, they aren’t 100% effective at preventing infection. In clinical trials and in real-world follow up studies, fully vaccinated individuals have gotten COVID-19, but illness has been mostly mild with very few hospitalizations related to those infections.
- What can I do now that I am vaccinated?
Vaccination provides excellent protection against COVID-19, and as more of the population is vaccinated, we’ll be able to slowly return to more normal life. A person is considered “fully vaccinated” two weeks after one dose of a single dose vaccine (Johnson & Johnson vaccine) or two weeks after the second dose of a two dose vaccine regimen (Moderna and Pfizer vaccines). CDC guidance was recently updated and reflects that fully vaccinated individuals may gather together indoors without masks and may gather with low-risk, unvaccinated individuals from one other household indoors without masks. Masks are not necessary in many outdoor situations, such as small gatherings or exercise. Additionally, travel within the U.S. does not require testing or quarantine if one is fully vaccinated, except for travelers to Hawaii.
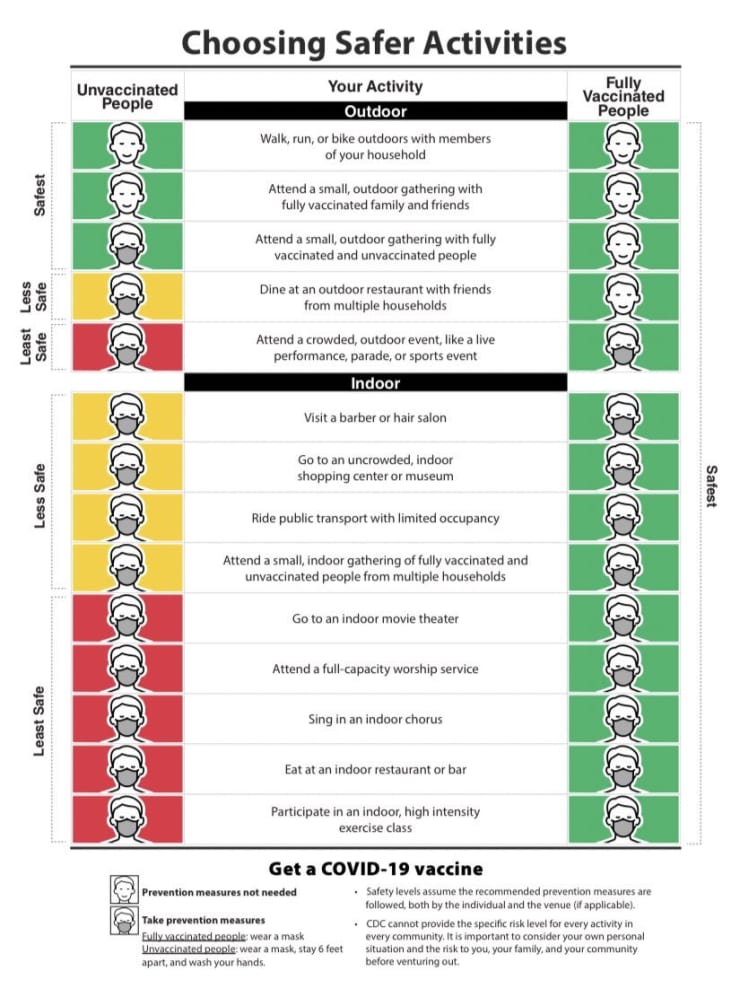
These are small steps forward, but much of our population is not yet fully vaccinated, and the virus is still circulating in our communities. Therefore, continued use of masks or face coverings and distancing when possible in indoor public places or crowded outdoor areas is advised. And, of course, don’t forget to wash those hands!
Those who are immunosuppressed, either from medications (such as those used to treat autoimmune conditions or prevent transplant rejection) or from medical conditions that cause immunodeficiency should continue more stringent precautions against exposure to COVID-19. In these individuals, vaccine response may be much less protective than in the general population.
- Is one vaccine better than another?
The short answer is “No”.
The longer answer is that there are advantages to each of the three FDA authorized COVID-19 vaccines, and those advantages go beyond simple comparisons of effectiveness. The Johnson & Johnson vaccine is much easier to transport and store and requires only a single dose, making it a good option for more remote locations or for those who may not follow through with a second shot. All of the available vaccines will contribute to decreased community spread of the virus and getting us back to a more normal life.
On the question of efficacy, the phase 3 clinical trials of the Moderna and Pfizer vaccines were done under very different conditions than the phase 3 Johnson & Johnson vaccine study. The mRNA vaccine studies were largely conducted during a period of lower virus transmission in the U.S., which could affect the infection rates in the study groups. The Johnson & Johnson study was conducted during the third “surge” of virus in the U.S. but also in centers world-wide, when there was emergence of more transmissible variants. Top-line efficacy numbers don’t reflect this complexity, but even in the different trial conditions, all vaccines are highly effective at preventing severe illness and death from COVID-19.
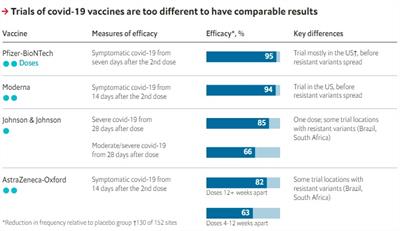
Recently, the U.S. paused administration of the Johnson & Johnson vaccine due to reports of a rare blood clotting complication, but has since resumed use of this vaccine after a thorough evaluation. While the risk of these events is very low, women under the age of 50 carry a slightly higher risk for these rare blood clots and may want to consider one of the mRNA vaccines if that choice is available.
- How long does the vaccine last and will I need a booster?
There is ample precedent for vaccine requirements in our society: schools require certain vaccines for children to go to school, some employers (particularly in healthcare) require annual flu shots for employees, and some countries require certain vaccines prior to travel. There are already many colleges and universities and healthcare facilities that have announced COVID-19 vaccine requirements for later this year.
While there is no current federal government proposal for a “vaccine passport” or similar requirement, some private businesses may elect to require proof of vaccination for customers or event-goers. Travel within the U.S. is not restricted by vaccine status, but other countries may require proof of vaccination for all visitors. The European Union is poised to reopen to vaccinated U.S. travelers this summer, and other destinations may follow suit.
- Will I need a vaccine passport or proof of vaccination to travel or go to in-person events?
There is ample precedent for vaccine requirements in our society: schools require certain vaccines for children to go to school, some employers (particularly in healthcare) require annual flu shots for employees, and some countries require certain vaccines prior to travel. There are already many colleges and universities and healthcare facilities that have announced COVID-19 vaccine requirements for later this year.
While there is no current federal government proposal for a “vaccine passport” or similar requirement, some private businesses may elect to require proof of vaccination for customers or event-goers. Travel within the U.S. is not restricted by vaccine status, but other countries may require proof of vaccination for all visitors. The European Union is poised to reopen to vaccinated U.S. travelers this summer, and other destinations may follow suit.
- Are there other vaccines that may get approval soon?
Expect to see the existing vaccines authorized for younger age groups before any additional vaccines are authorized for use in the U.S. The Astra-Zeneca vaccine is approved in the U.K. and E.U., and may eventually be authorized in the U.S., but as of now the doses purchased by the U.S. government are being shared with other countries and are not allocated for use here. Novavax also has a COVID-19 vaccine in clinical trials; this is a protein adjuvant vaccine, a different vaccine mechanism than the mRNA and vector vaccine types.
The good news is that the U.S. currently has enough vaccine supply for its adult population with the existing authorized vaccines. Additional vaccine candidates under study will help to meet global and future needs.
