Welcome to PROLIFIC

Biomarkers may hold the key to PF
PROLIFIC aims to guide and streamline the development of targeted treatment strategies for patients with pulmonary fibrosis. By developing tests to detect biomarkers in patients, PROLIFIC will study the early indicators of a drug’s activity. With the long-term goal of finding biomarkers that can predict which patients would benefit the most from a particular treatment, PROLIFIC’s efforts will enable physicians to better tailor therapeutic treatments for patients.
Consortium members have agreed to focus their research on 12 biomarkers in four key categories:
- Epithelial damage
- Fibrosis
- Inflammation
- Thrombosis
Researchers will screen samples from the PFF Registry to identify and validate blood protein biomarkers.
PROLIFIC is open to new members. Pharmaceutical and biotech companies, patient advocacy and non-profit organizations, and academic institutions are invited to inquire.Partners streamline discovery
The Pulmonary Fibrosis Foundation (PFF) and Bristol Myers Squibb launched PROLIFIC to simplify the research process for all by promoting cooperation among competitors to create a company-agnostic assay for use during drug development.
The PFF Care Center Registry has played a key role in PROLIFIC’s efforts. Once a multi-assay panel has been developed, consortium members will test patient samples from the Registry’s biorepository to validate their results.
Thank you to Myriad RBM for development work on a new multiplex biomarker assay on behalf of the PROLIFIC Consortium and to the Statistical Analysis of Biomedical and Educational Research Group (SABER) at the University of Michigan for development of the statistical analysis plan and data coordination of the PFF Registry.

Publications and latest news
A first for IPF biomarkers: PROLIFIC risk score nears FDA qualification
January 2026
Article
Source: touchRESPIRATORY
First IPF Biomarker Clears Initial FDA Step Toward Qualification
December 2025
Press Release
Source: Pulmonary Fibrosis Foundation
Development of a Multi-biomarker Risk Score Based on Serum Proteins by the Prognostic Lung Fibrosis Consortium (PROLIFIC)
August 2024
Abstract
Poster
Authors: P. Schafer, J. Rassa, A. Seyda, S. Hersey, D. Eisinger, J. Melin, R. Bencher, M. Neighbors, M. Mouded, S. Turner, M. Decaris, Y. Bauer, C.L. Nickerson-Nutter, D. Ball, J. Larson, J. Swaney, S. R. Lim10, A. C. MacKinnon, F. Marabita, J. Mefford, S. Staszak, A. Martin-Schwarze, Z. Zheng, P. Song, J. A. Lasky
Source: ERS 2024
Development of a Multi-biomarker Assay for Serum Proteins by the Prognostic Lung Fibrosis Consortium (PROLIFIC)
May 2024
Abstract
Poster
Author: P. Schafer, S. Hersey, J. Rassa, A. Seyda, D. Eisinger, J. Melin, R. Bencher, M. Neighbors, M. Mouded, A. Martin-Schwarze, S. Turner, M. Decaris, Y. Bauer, C.L. Nickerson-Nutter, D. Ball, J. Larson, J. Swaney, S. R. Lim, A. C. MacKinnon, F. Marabita, J. Mefford, S. Staszak, J. A. Lasky
Source: ATS 2024
Bridge Biotherapeutics Joins PROLIFIC, the Prognostic Lung Fibrosis Consortium
July 2023
Press Release
Source: Bridge Biotherapeutics, Inc.
Pulmonary Fibrosis Foundation Launches Industry Consortium To Identify Biomarkers In Pulmonary Fibrosis
March 2020
Press Release
Source: Pulmonary Fibrosis Foundation
What are biomarkers?
Biomarkers—molecules that reveal whether a biologic process is happening inside a cell—can be useful tools in understanding, treating, or even preventing disease. Biomarkers can potentially identify people who:
- Have a specific disease.
- Are at risk of developing a disease.
- Might have better or worse disease outcomes.
- Can receive a treatment safely.
- Might respond better to one treatment than another.
In order to harness the power of biomarkers for the chronic, fatal lung disease pulmonary fibrosis, scientists must first:
- Identify the biomarker.
- Develop a test, or assay, that reliably indicates the presence, absence, or change in amount of a biomarker.
- Correlate the biomarker with a consistent outcome in the cell.
- Validate that assay by testing it in large, multicenter studies including diverse groups of patients.
Researchers have identified dozens of blood proteins that could be potential biomarkers in pulmonary fibrosis, but studying each one separately is too resource intensive. Members of PROLIFIC have identified 12 promising potential biomarkers, across four main categories, to explore collaboratively. Click through the categories below to explore more about each biomarker.
Consortium members have agreed to focus their research on 12 biomarkers in four key categories
Epithelial Damage
Epithelial cells line the alveoli, or air sacs, of the lungs. These
cells allow materials to pass through by diffusion and filtration.
Damaged epithelial cells lose their permeable qualities, blocking the
passage of air through the lungs into the blood.
Fibrosis
Fibroblasts synthesize collagen proteins used in the body’s connective tissues. These proteins help maintain body structures and can be important for wound healing. Scar tissue forms if the body’s normal cell repair process continues too long. In idiopathic pulmonary fibrosis (IPF), the over-formation of scar tissue results excessive stiffness in the lungs’ extracellular matrix.
Inflammation
Inflammation is part of the body’s normal healing process. But when inflammation lasts too long, it can lead to tissue damage.
Thrombosis
Thrombosis, or the formation of blood clots, can occur with pulmonary fibrosis. Studies have shown that people with IPF are much more likely to develop blood clots in their lungs than others, but the exact mechanism behind this elevated risk remains unclear.
Impacts of damage on lung tissue
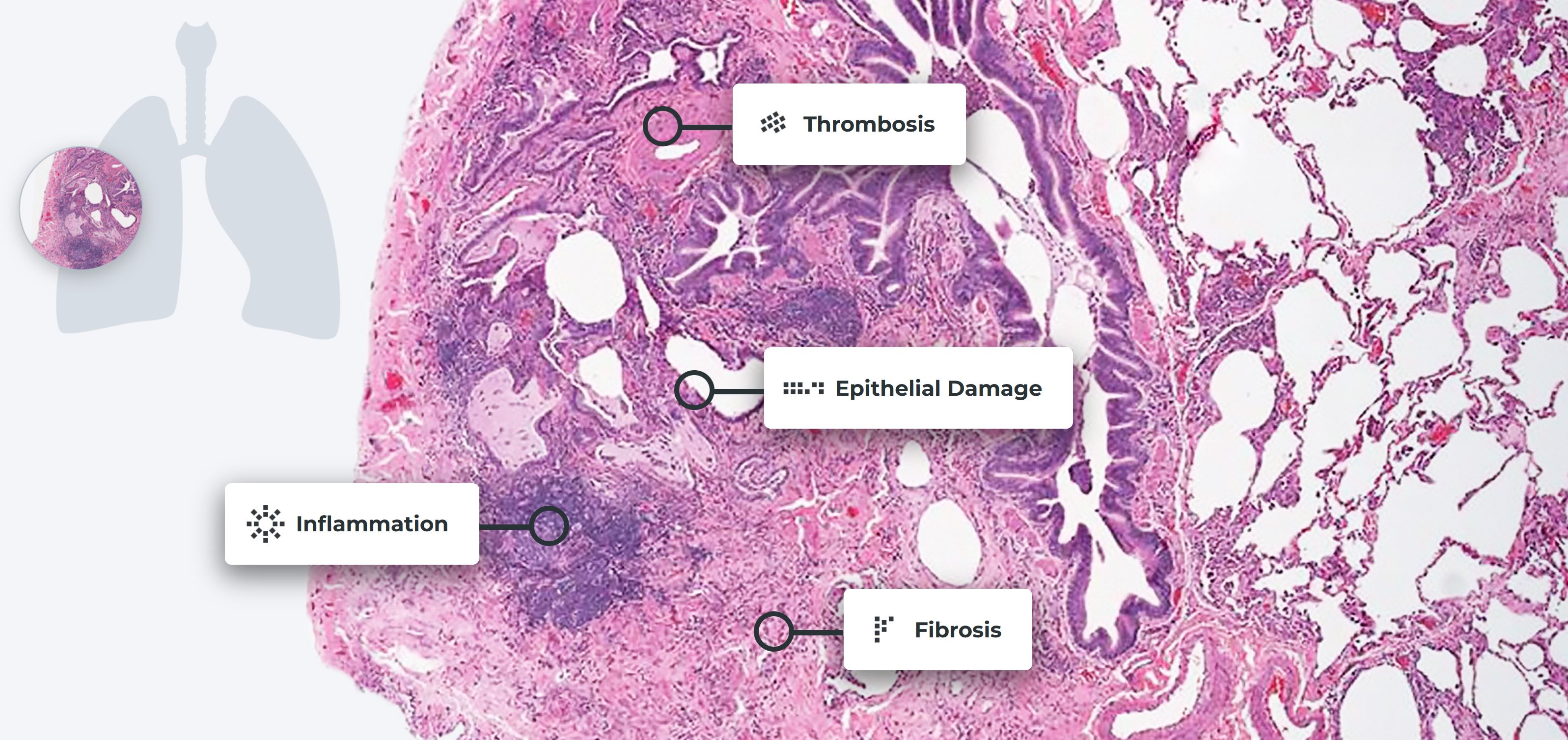
Magnified view of lung tissue revealing the presence of scleroderma lung disease. Reproduced with permission of the © ERS 2021: European Respiratory Review 22 (127) 6-19; DOI: 10.1183/09059180.00005512 Published 28 February 2013.
You can participate in discovery. Contact us today!
Research organizations and other industry partners are welcome to join PROLIFIC. Use the contact form below to get in touch.
If and when a biomarker assay or assays resulting from PROLIFIC’s work receives regulatory approval, contributing members will be invited and encouraged to use the new panel.